
8 Innovative Ideas for Eco-Friendly Cosmetic Packaging
2024-11-05

The US Food and Drug Administration (FDA) has strict regulations on cosmetics packaging. A slight oversight can lead to the seizure of goods, not only causing economic losses but also delaying the product launch time and affecting the brand’s reputation. Richpack, with its rich industry experience, has accumulated valuable practical experience in assisting numerous brands to complete FDA 21CFR and ECOCERT certifications. Today, we will take you deep into the easily overlooked packaging certification details to help you avoid the many pitfalls on the way to international expansion.
To successfully overcome the numerous obstacles in cosmetics export and pass the strict FDA review smoothly, clear and well-organized implementation steps are the key. Next, I will elaborate on each indispensable step in detail, so that you can be well-informed and no longer confused when dealing with the complex packaging certification tasks.
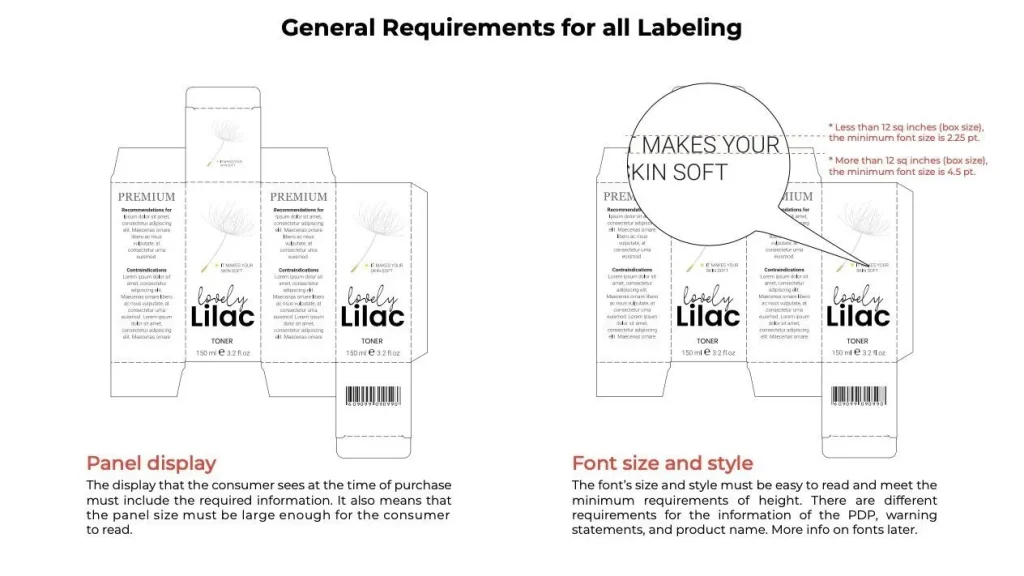
First and foremost, you need to have a comprehensive understanding of the specific regulations regarding cosmetics packaging in FDA 21CFR. This includes requirements for the safety of packaging materials, the information that must be included on labels, and regulations on the compatibility between jewellery boxes and products. For example, Part 700 of 21CFR has clear requirements for the ingredient labeling of cosmetics. All ingredients must be listed in descending order of content and use the standard INCI (International Nomenclature of Cosmetic Ingredients) names.
When choosing cosmetics packaging materials, a strict safety assessment must be carried out. The FDA has strict restrictions on packaging materials that come into direct contact with cosmetics. For instance, certain chemical substances in some plastics may migrate into cosmetics, posing potential threats to consumers’ health. Our Richpack has a professional material analysis team that can assist you in screening out packaging materials that meet FDA standards, such as specially treated glass and plastics that meet food-contact levels, to ensure the safety of packaging materials.
Product labels are one of the key focuses of FDA inspections. The label should include the product name, brand name, net content, ingredients, usage instructions, and warnings. Make sure all the information is accurate, clear, and in English. For example, if your product contains ingredients that may cause allergic reactions in some consumers, it must be clearly indicated on the label. We have professional designers who are familiar with FDA label requirements and can help you create a compliant and eye-catching label design.
If your brand aims to highlight its commitment to environmental protection and sustainable development, ECOCERT certification can be a great addition. ECOCERT is an internationally recognized certification body for organic and natural products. To obtain this certification, you need to ensure that your packaging materials meet certain environmental standards, such as being made from recycled or biodegradable materials. The application process involves submitting detailed documentation about your packaging materials, production processes, and environmental management systems. We can provide guidance throughout the ECOCERT certification process, from material selection to documentation preparation.
Merely knowing the basic steps is not enough. In the actual operation process, some practical tips and best practices can help you achieve twice the result with half the effort and reach your goals in a more efficient and high-quality way.
The regulations of the FDA and other certification bodies are constantly evolving. It is essential to stay updated with the latest regulatory changes. Subscribe to industry newsletters, follow regulatory agency websites, and participate in relevant seminars or webinars. This way, you can ensure that your packaging certification strategies are always in line with the latest requirements.
Before shipping your cosmetics to the US, conduct thorough pre-shipment inspections. Check the packaging materials, labels, and product quality to ensure everything is in compliance. You can also consider hiring a third-party inspection agency with expertise in FDA regulations to conduct an independent inspection. This can help you identify and rectify any potential issues before they lead to costly problems at the US customs. At Richpack, we have in-depth knowledge of these inspection procedures and can offer useful advice.
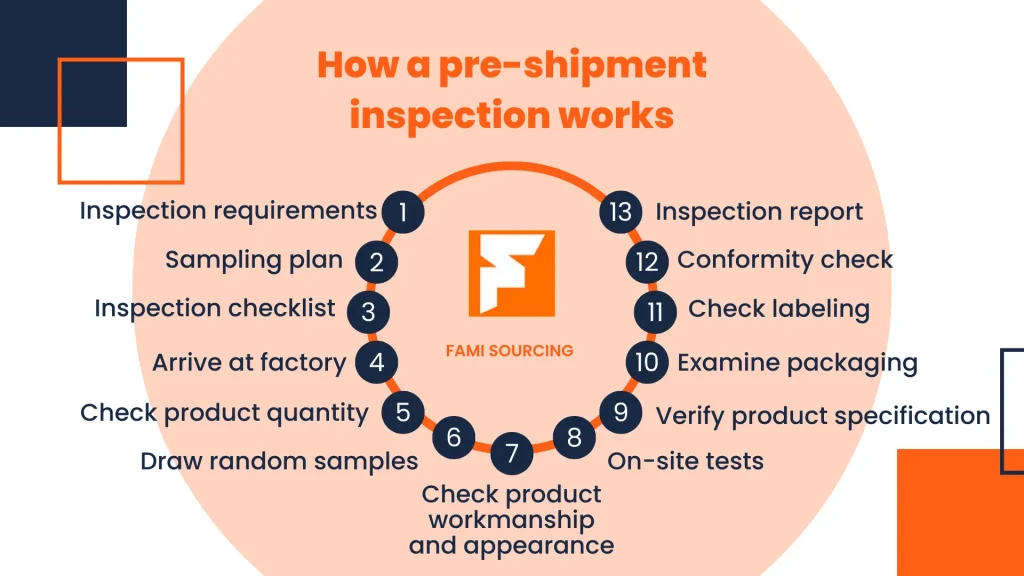
A reliable packaging supplier is crucial for ensuring the success of your cosmetics export. Work closely with your supplier, communicate your requirements clearly, and ensure they are aware of the FDA regulations. At Richpack, we not only provide high-quality packaging solutions but also offer continuous support and guidance to our clients throughout the entire process, from design to production.
On the journey of cosmetics export, there are various hidden reefs and pitfalls. Understanding the common mistakes and pitfalls and knowing how to avoid them are important guarantees for ensuring the smooth customs clearance of your goods and the steady development of your brand. The following will deeply analyze the common problems that are likely to lead to FDA seizures and the corresponding solutions for you.
One of the most common mistakes is incomplete or incorrect labeling. This can include missing ingredient information, using non-standard ingredient names, or unclear usage instructions. To avoid this, double-check all the label information before finalizing the design. It is also advisable to have a legal expert review the label to ensure compliance with all relevant regulations. Richpack can connect you with professionals who are well-versed in label compliance if needed.
Using packaging materials that do not meet FDA standards is another major pitfall. Some brands may choose cheaper materials without fully understanding the regulatory requirements, which can lead to product recalls or seizures. Always conduct thorough research on packaging materials and consult with professionals like Richpack to ensure the materials you choose are compliant. You can learn more about suitable packaging materials by exploring the green material options on our website.

If your brand is targeting the eco-friendly market and plans to obtain ECOCERT certification, neglecting the certification process can be a costly mistake. Some brands may underestimate the complexity of the certification requirements and rush into production without proper preparation. To avoid this, start the certification process early, work closely with a certification consultant, and ensure all aspects of your packaging and production processes meet the ECOCERT standards. Richpack has experience in guiding clients through the ECOCERT process and can offer detailed assistance.
FDA regulations are in place to protect consumers, and compliance is not only a legal requirement but also a way to build trust with your customers. Whether you are a small-scale beauty brand or a large-scale multinational company, Richpack is here to support you with our expertise in packaging design, material selection, and certification guidance. If you have any questions or need further assistance, feel free to contact us. Start applying these strategies today and take the first step towards successful cosmetics export! If you want to explore more about packaging designs, you can read here for some inspiring ideas that can also be adapted to cosmetics packaging.

According to a survey, 72% of American consumers agree that good-looking packaging, like beautiful makeup packaging, plays a role in their buying decisions. In the competitive beauty market, for business owners who want to make their cosmetic brand stand out, the effective way is to design packaging that draws customer’s attention and reflects your brand’s identity. In this article, we… Continue reading 4 Packaging Certification Details That Lead to FDA Seizures

Cosmetics is almost every girl has, it can be said that cosmetics is also a very good sales of products, young girls in the purchase of cosmetics not only to buy good quality, in the selection of the appearance of the box is beautiful or not is also a factor affecting whether they buy, therefore,… Continue reading 4 Packaging Certification Details That Lead to FDA Seizures

richpack richpack · Unboxing Delight Crafting Memorable Experiences That Build Your Brand The unboxing experience has evolved from a simple act of opening a package to a crucial brand touch point that can shape customer perception. It’s an extension of your brand’s narrative and a chance to forge a deeper connection with your audience. Richpack’s custom Jewellery… Continue reading 4 Packaging Certification Details That Lead to FDA Seizures

Elegant Tray Display Solutions for Showcasing Products – Discover Customizable Display Trays for Retail and Event Presentations with Richpack

Luxury Perfume Packaging with Brand Logo for Designer Fragrance Brands | Ideal for Brands Seeking High-End, Branded Packaging Solutions

Luxury Perfume Packaging with Velvet Lining | Designed for Premium Perfume Brands Needing Elegant and Protective Packaging for Exclusive Fragrances

Custom Cosmetic Box Packaging for Luxury and Retail Brands – High-Quality Cosmetic Packaging Boxes Wholesale for Unique and Sustainable Packaging Solutions
View More
Custom Logo Paper Box Makeup Brush Set Cardboard Cosmetic Brush Packaging Coating Beautiful
View More
Custom Small Batch Cosmetic Boxes for Beauty Retailers | Ideal for Small Businesses Needing Tailored, High-Quality Packaging in Limited Quantities
View MoreJust submit your email to get exclusive offers (reply within 12 hours)